
Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. Images © Murray Robertson 1999-2011 Text © The Royal Society of Chemistry 1999-2011 Political stability of top reserve holderĪ percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. The higher the value, the larger risk there is to supply.Ī percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The percentage of the world reserves located in the country with the largest reserves. The higher the value, the larger risk there is to supply. The percentage of an element produced in the top producing country. Low = substitution is possible with little or no economic and/or performance impact Medium = substitution is possible but there may be an economic and/or performance impact High = substitution not possible or very difficult. The availability of suitable substitutes for a given commodity. A higher recycling rate may reduce risk to supply. The percentage of a commodity which is recycled. The number of atoms of the element per 1 million atoms of the Earth’s crust. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems.ĭata for this section been provided by the British Geological Survey.Īn integrated supply risk index from 1 (very low risk) to 10 (very high risk). Where more than one isotope exists, the value given is the abundance weighted average.Ītoms of the same element with different numbers of neutrons. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an atom relative to that of carbon-12.
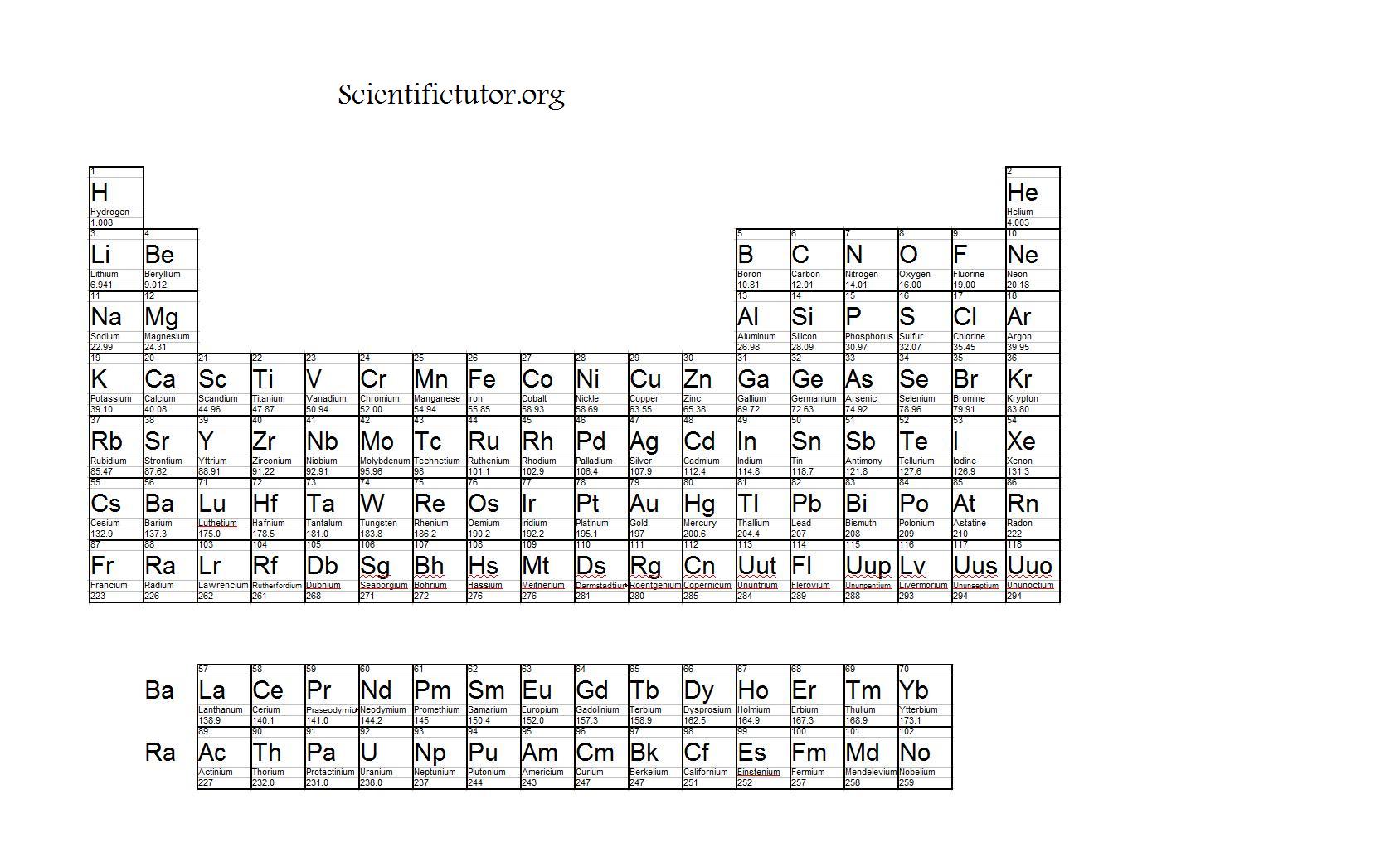
The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.ĭensity is the mass of a substance that would fill 1 cm 3 at room temperature. The temperature at which the liquid–gas phase change occurs. The temperature at which the solid–liquid phase change occurs. The arrangements of electrons above the last (closed shell) noble gas. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The atomic number of each element increases by one, reading from left to right.Įlements are organised into blocks by the orbital type in which the outer electrons are found. Members of a group typically have similar properties and electron configurations in their outer shell.Ī horizontal row in the periodic table. We have excluded elements above 108 on this periodic table for the simple reason that the larger elements don’t really exist in the real world.A vertical column in the periodic table. You might find a table of elements showing element 115 or even 118 elements total. There is a Netflix documentary about this topic entitled Bob Lazar: Area 51 & Flying Saucers. The elements above 92 are strange, like for example element 115 that was reportedly found at Area 51. The parenthesis are a scientific way of saying this number is guesstimated, at best, and we really don’t know much about these elements because they are artificial and tend to disintegrate rapidly. You might note in the periodic table the mass numbers are in parenthesis for elements 93 and above. At least you won’t be finding any in this lifetime. As such, elements above 92 don’t really exist, except under special laboratory conditions. All elements above atomic number 92 are created artificially in laboratories, and generally they are extremely unstable and tend to disintegrate rapidly. As such, it’s highly unstable and unable to exist for very long. It is element 94, making it bigger than uranium. In 1940, a new element, plutonium, was created in a laboratory.

Element 92 is uranium, the biggest element that occurs naturally on Earth. Before the year 1940, it was believed that only 92 elements existed. (See the original work of Dmitri Mendeleev here.) At that time, scientists were still discovering new elements almost every year. The original table of the elements, credited to Dmitri Mendeleev in 1871, had only 56 elements. The table of elements shown here has 108 elements.


 0 kommentar(er)
0 kommentar(er)
